Managing Monitoring Rounds
All your active Monitoring Rounds will be listed in the "Your Active" screen as below
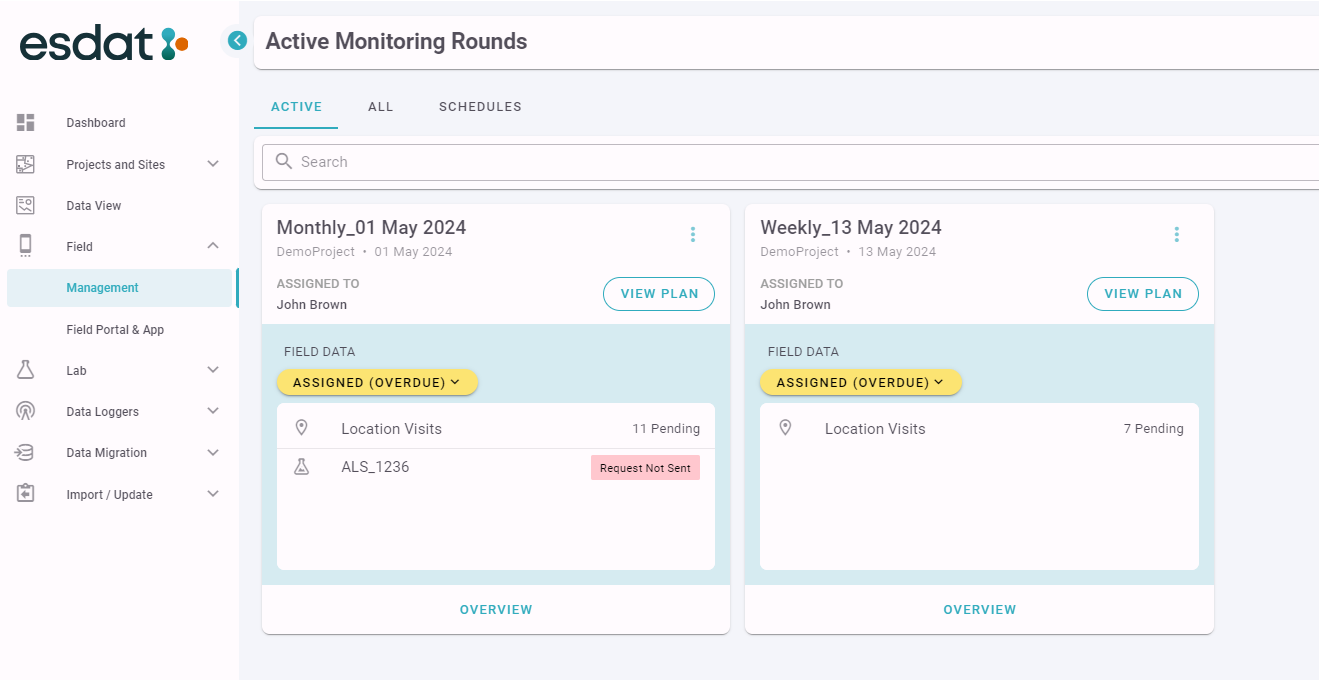
From this screen a Manager or Supervisor can monitor the data that is being collected in the field in the ESdat Field App. For each active Monitoring Round the Manager or Supervisor can immediately see how many Location Visits are still pending, which Laboratory Submissions have been created and their status (S = Sent, NS = Not Sent. A tooltip will display when hovering over the value giving the full description.)
From this screen the Manager or Supervisor can navigate to view the following for a specific Monitoring Round:
- The Monitoring Round Plan
- An Overview of the status, the field data collected and work remaining.
- Each Laboratory Submission.
The Overview screen looks as shown in the image below:
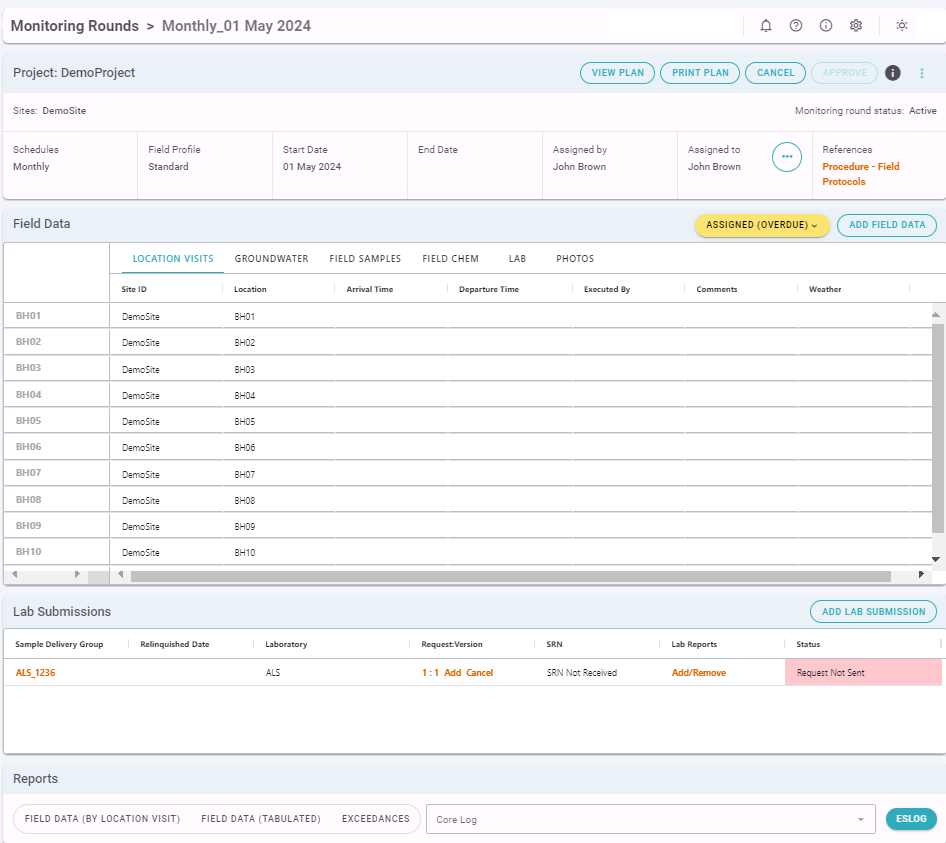
The Field Data Section Lists each Location Visit in the Monitoring Round. An overview of the data collected can be viewed, or each Location Visit can be clicked in order to edit values. Completed Location Visits are shown in green. Location Visits that were planned but are not complete are shown in grey.
The Laboratory Submissions section shows the Status of each Laboratory Submission in the Monitoring Round. Corresponding Sample Receipt Notification (SRN) and Laboratory Report information (when received) can also be accessed from here.
At the bottom of the page are links to some reports that are useful for outputting Monitoring Round information to PDF or other formats.
Once a Field Work Status has been set as completed and Laboratory Reports received and approved for each Laboratory Submission the Monitoring Round can be marked as Approved, and it will be removed from the "Your Active" list.
Laboratory Submissions
Laboratory Submissions are always associated with a Monitoring Round, and can be created from Plans, from a copy of a previous Submission, or as a new/blank Submission.
Typically Laboratory Submission information will be populated by field staff using a field device with the ESdat Field App and the data sync directly back to ESdat. Field Staff can submit a simplified Chain of Custody document to accompany the samples to the Laboratory (excluding Analysis Request and other information)
The Manager or Supervisor in the Office can review and update Sample Details and Analysis Request information prior to sending an electronic Laboratory Request to the Laboratory.
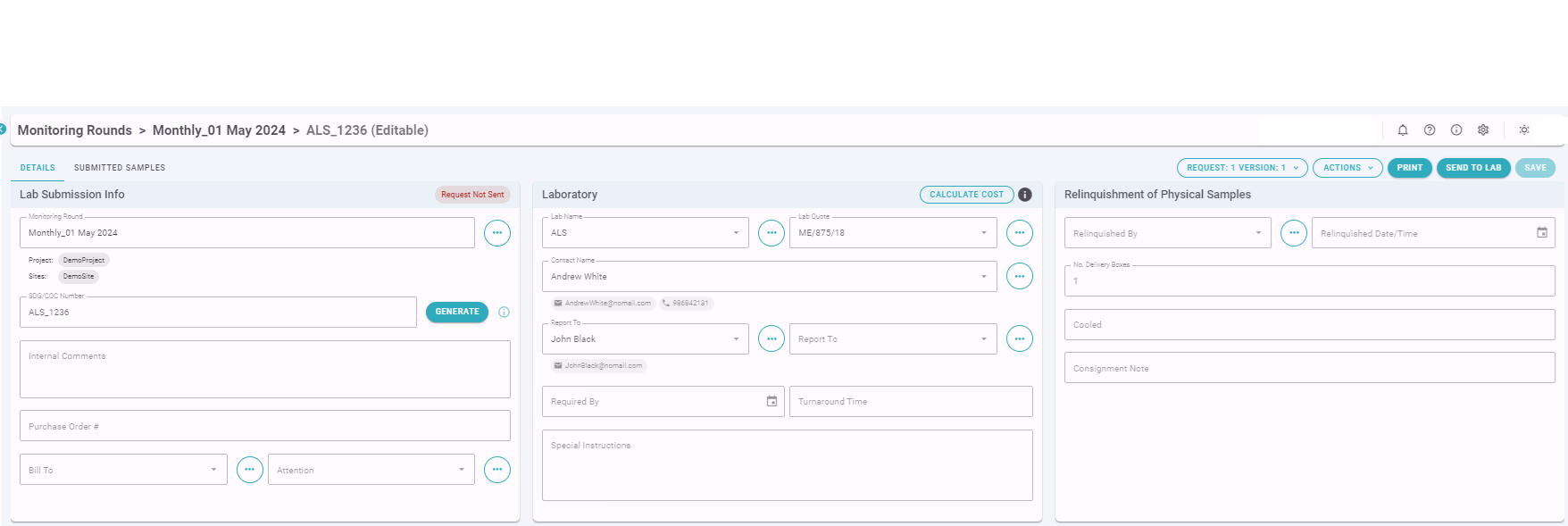
It is important that the SDG/COC Number here matches the COC Number sent to the laboratory with the actual samples.
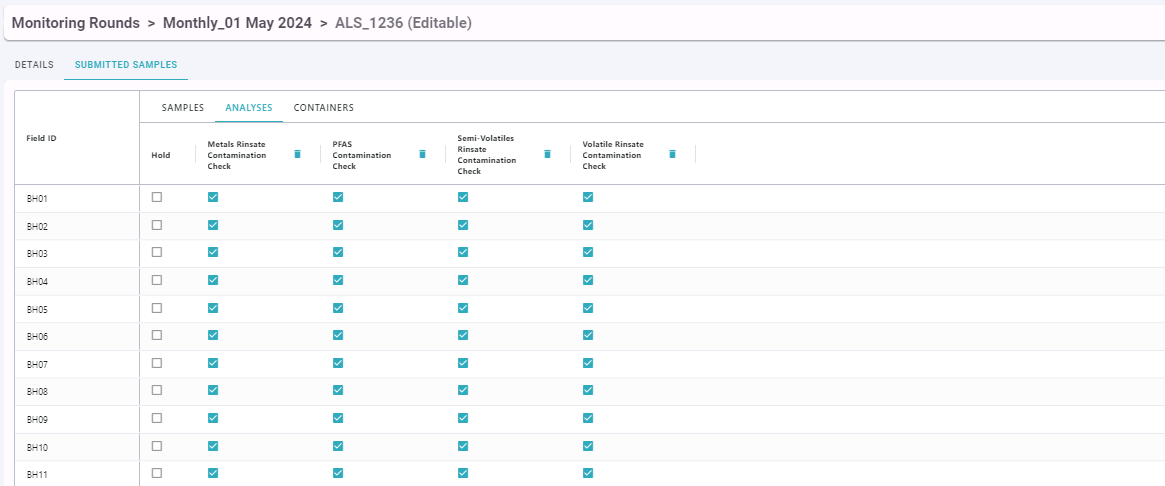
Analyses that can be selected are sourced from the Lab Quote referenced in the Lab Submission. Organisations can also create their own internal list of Analyses to use if appropriate Analyses are not available in the Lab Quote.
Once reviewed a Laboratory Request can be sent to the Laboratory. Laboratory information is sent in two formats:
- a printable copy that looks like a traditional Chain of Custody document.
- An xml format so that laboratories can automate import of Chain of Custody / Sample and Analysis information.
If changes are needed after the first Laboratory Request was sent users can create and submit a new version of that Laboratory Request.
If a second round of analysis is to be requested on some samples users can create a second (or subsequent) Laboratory Request based on the same Laboratory Submission. When a new request is created the request number is incremented and the same Lab Submission and Sample information is retained; so the new Analyses can be selected.
