QA Reports
Quality Assurance is a critical step in receipt and Acceptance of Laboratory Data to ensure data integrity.
Assessing the quality of a Lab Report is time critical (i.e. if you need to follow up with the Lab, reanalyze or reissue results), so it is recommended that QA assessment will be completed as Lab Reports are imported.
QA Assessment requires that QA Samples are specified into the Sample Type field either during fieldwork in the Field App or PLog or after receipt of the Laboratory Report.
QA Reports that ESdat provides include:
- Sample Frequency Validation
- Field or Interlab Duplicates
- Laboratory Duplicates
- Field Blanks
- Laboratory Blanks
- Matrix Spikes
- Trip Spikes
- Surrogates
- Lab Control Samples
- Standard Reference Materials
- Certified Reference Materials
- Inorganic Logic Checks
- Dilution Factors
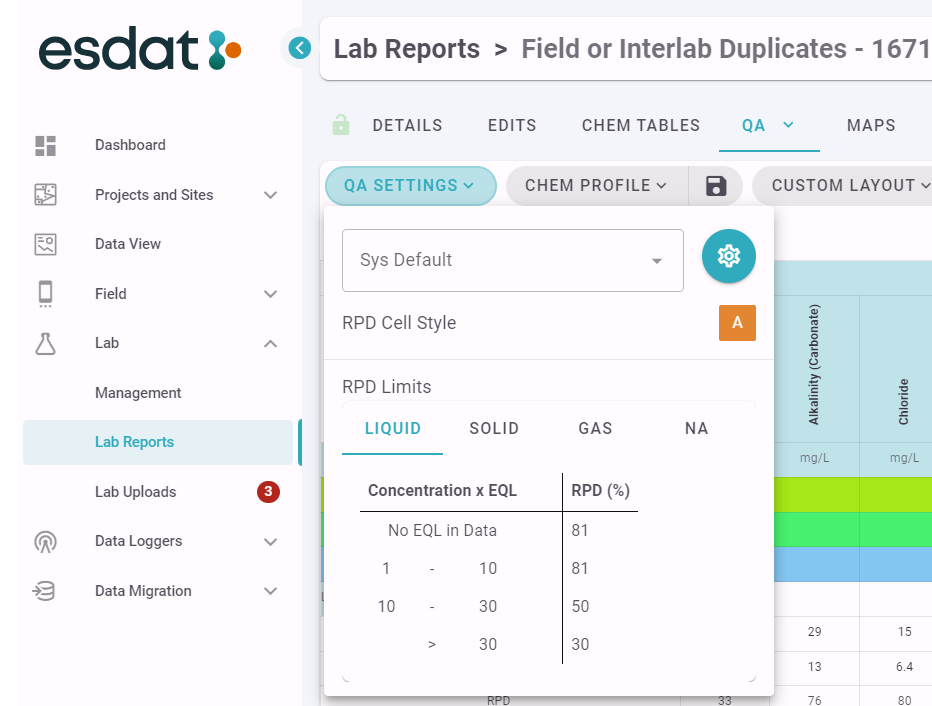
The settings for each QA report can be seen on the top right menu as shown below.
Users can create different sets of QA Settings for use in different scenarios. A full description of the QA Settings that can be applied is described in qa-settings
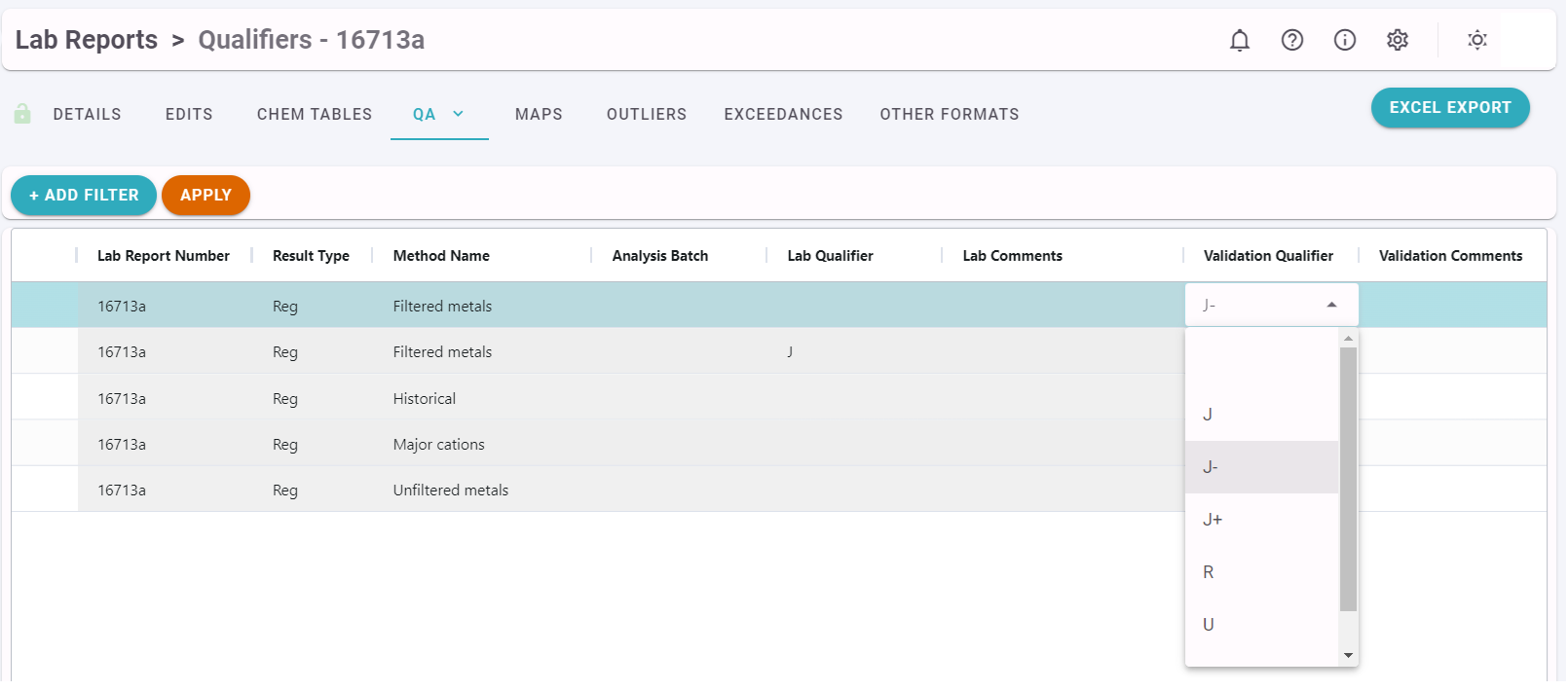
Qualifiers
The Qualifiers QA report allows users to bulk apply Qualifiers to Laboratory Results based on a Method and Analysis Batch.
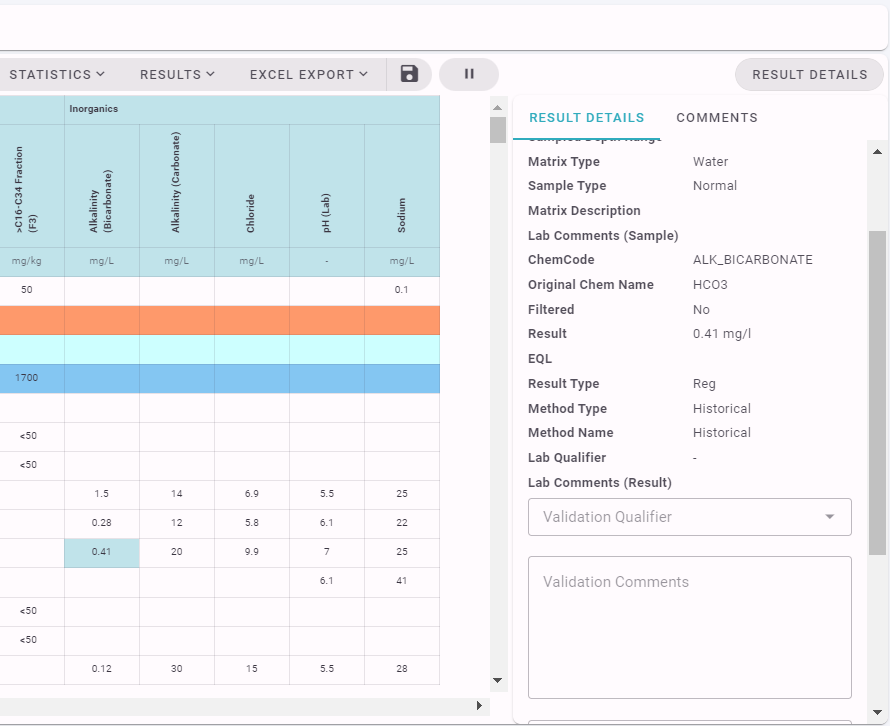
In addition, Qualifiers can be assigned against an individual result on a Chemistry Table by clicking on a result, viewing the Result Details and Comments Panel, and selecting a Qualifier.